
Covalent bond definition
n., koʊˈveɪlənt bɑnd
A type of chemical bond wherein two or more atoms share one or more electron pairs
Table of Contents
Covalent Bond Definition
What is a covalent bond? In chemistry and other fundamental science fields, a covalent bond is defined as a chemical bond wherein two or more atoms share one or more electron pairs.
How about in biology? What does covalent bond mean? In biology, the covalent bond is one of the three major types of chemical bonds that are biologically important; the other two are ionic bonds and hydrogen bonds.
The covalent bond, though, is distinct from these two bonds in such a way that the electrons are shared between atoms, ions, or molecular constituents of a biological compound.
Etymology: from -co, meaning “together” and “valent”, “valence”, from Latin valentia, meaning “strength” or “capacity”.
Synonyms: molecular bond.
Covalent Bond Characteristics
What are covalent bonds? They are made up of simultaneous attraction of nuclei for electron pairs. These electrons that are getting shared between nuclei or among atoms are one of the main characteristics that define a covalent bond. Rather than the process of giving and receiving, covalent bonding entails the sharing of electrons in pair(s).
The electrons travel between the nuclei of the atoms being held together and kept at a stable distance apart. Referred to as an electron pair (also called a bonding pair or Lewis pair), the pair will consist of two electrons in the same molecular orbital but have opposite spins. Covalent bond forms only on orbitals with unpaired electrons. These two orbitals will overlap and form a covalent bond. (Ref.1)
The carbon atom, for instance, has four valence electrons in its outer shell. Rather than lose the electrons it shares them with other atoms as long as they have comparable electronegativities for covalent bonding to occur (refer to the figure below). Aside from carbon, the most common forms of atoms in a living system are hydrogen, oxygen, nitrogen, phosphorus, oxygen, and sulfur.
Each of them has an outermost orbital with a characteristic number of electrons. Hydrogen has one, carbon has four, nitrogen and phosphorus have five, oxygen and sulfur have six. Their atoms can readily form covalent bonds with other atoms. Hence, they rarely exist as isolated entities. (Ref. 2)
Physical Properties of Covalent Bond
A pair of electrons between atoms or molecules that covalently bond will result in the sharing of electrons, not giving or taking electrons as what is expected to happen in an ionic bond. The explanation as to why they share rather than lose or take electrons is because of electronegativity.
Electronegativity is a physical property of a chemical bond that helps to explain how an atom will attract electrons towards it. In a covalent bond, the electronegativities of the two atoms are neither strong nor weak but somewhat the same. The electronegative forces between them are somewhat neutral and so they end up simply sharing their electrons. See the picture below.
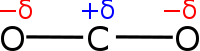
Compounds that have covalent bonds have been shown to have low boiling point and melting points. They have low conductivity. They do not conduct electricity. Compounds that have ionic bonds tend to be more conductive as their constituents are charged particles capable of transporting electrons.
These complicated properties are associated with the Lewis theory of covalent bonding. According to this theory, the atoms tend to share their valence electrons. This allows each atom to create its own octet and the result is enhanced stability. (Ref.3)
Covalent bond vs. Ionic bond
While ionic bonds involve the transferring of electrons from one to another, covalent bonds involve the sharing of electrons between atoms. It is argued that there is no pure ionic bonding.
An ionic bond has a covalent character in it. The bond is said to be ionic rather than covalent when there is a large difference in electronegativity between the two atoms. This is what happens between sodium and chlorine atoms.
The sodium ion bonds with the chlorine ion and the result is sodium chloride. (Ref. 4) Refer to the table below for more info and facts. It will also describe the differences between an ionic bond and a covalent bond.
| Ionic bond | Covalent bond | |
|---|---|---|
| Formation | An ionic bond is formed by the transfer of electrons between atoms of one metal and one non-metal. The compound that results in an ionic bond is called an ionic compound. | A covalent bond is formed by the sharing of electrons between atoms of two elements, such as between two non-metals. |
| Electronegativity (i.e. the ability of an atom to attract electrons towards it) | The strong electronegativity of one atom attracts electron(s) from another atom. The atom that is strongly electronegative can gain electron(s) and becomes an anion. Conversely, the atom that is weakly electronegative loses electron(s) and becomes a cation. | The electronegativity of one atom is not strong enough or is somewhat the same as that of another atom, thus, the atoms tend to share rather than get or give electrons to achieve a stable electron configuration. |
| Salt formation | The electrostatic attraction between anion and cation forms an ionic compound. Many of the ionic compounds are referred to as salts since they can be formed by the neutralization reaction between a base (e.g. OH-) and an acid (H+). | The bond that forms between the atoms does not result in the formation of a salt. |
| State at room temperature | Solids, with a crystallographic lattice | Solids, liquids, gases |
| Types | – | Single bond, double bond, triple bond, (depending on sigma and pi bonds involved) |
Covalent bond vs. Hydrogen bond
As the name implies, a hydrogen bond is a chemical bond wherein hydrogen serves as a bridge between two atoms. Similar to a covalent bond, the hydrogen bond is a common chemical bond in organic compounds, particularly nucleic acids and proteins. The hydrogen bond is responsible for the formation of secondary and tertiary structures of nucleic acids and proteins.
One of the major distinctions between a covalent bond and a hydrogen bond is the electronegativities of the atoms involved. In a covalent bond, the electronegativities of the two atoms are comparable. Conversely, hydrogen bond forms when the slightly positive hydrogen atom of a polar covalent bond forms an electrostatic link with the more electronegative atom of a polar covalent bond in the same or another molecule.
Types of Covalent Bonds
sigma (σ) bond vs pi (π) bond
There are different ways to classify covalent bonds. For example, they may be classified as sigma (σ) bond or pi (π) bond based on the covalent formation and interaction. σ bonds are formed by head-on overlapping between atomic orbitals. It is the strongest type of covalent bond. π bonds are covalent bonds formed when the two lobes of an orbital on one atom laterally overlap the two lobes of an orbital on another atom.
Single bond vs double bond vs triple bond
A covalent bond may be single, double, or triple. A single bond (-) is when the atoms share two electrons. It is usually a sigma (σ) bond. For example, a water molecule has two hydrogen atoms and a central oxygen atom that are held together by two single covalent bonds. Each hydrogen atom shares a pair of electrons with the oxygen atom.
A double bond (=) is the sharing of four electrons between two chemical elements. The stronger σ bond and the weaker pi (π) bond often make up a double bond. Examples of double bonds are those found in alkenes, azo compounds, imines, and sulfoxides.
When the covalent bond involves six electrons between two atoms, it is a triple bond. A triple bond (≡) usually has one σ bond and two π bonds. An example is a triple bond in alkynes, cyanides, isocyanides, and carbon monoxide.
The single bond is the weakest of the three since there is only one bond that connects two atoms together.
More Covalent Bond Examples
Organic compounds
Covalent bonds, similar to other chemical bonds, help to form a chemical compound, such as an organic compound that contains carbon atoms that are generally bound covalently to other atoms. Organic compounds are of vital importance because all living things are based on these compounds. Examples of organic compounds are carbohydrates, lipids, proteins, and nucleic acids that are involved in various metabolic processes.
Diatomic hydrogen molecule

Description: a 3D structure of the diatomic hydrogen molecule
Diatomic hydrogen molecule, H2, is a combination of two hydrogen atoms. A single covalent bond holds the two atoms together. Thus, it can be written as H-H. The dash (-) signifies the covalent linkage between the two hydrogen atoms.
Water molecule

A water molecule consists of one centrally-located oxygen and two smaller hydrogen atoms attached to the oxygen by a covalent bond. This results in a partially positive pole and a partially negative pole. This makes water a polar molecule. Apart from the covalent bond, though, a water molecule can form a transient hydrogen bond with a nearby water molecule.
See also
References
- Covalent Bonding | Chemical Bonding | Siyavula. (2020). Siyavula.Com; Siyavula. https://www.siyavula.com/read/science/grade-10/chemical-bonding/06-chemical-bonding-03
- Lodish, H., Berk, A., S Lawrence Zipursky, Matsudaira, P., Baltimore, D., & Darnell, J. (2013). Covalent Bonds. Nih.Gov; W. H. Freeman. https://www.ncbi.nlm.nih.gov/books/NBK21595/
- The Covalent Bond | Boundless Chemistry. (2020). Lumenlearning.Com. https://courses.lumenlearning.com/boundless-chemistry/chapter/the-covalent-bond/
- Fundamentals of Chemical Bonding. (Jun 23, 2019). Retrieved from https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Fundamentals_of_Chemical_Bonding
©BiologyOnline. Content provided and moderated by BiologyOnline Editors.


