In the previous post, we mentioned that carbohydrates exist mainly in their cyclic forms when 5- or 6-membered rings are possible. Remember also that cyclic carbohydrates are hemiacetals which can be further transformed into acetals. The acetals of monosaccharides are called glycosides – acetals with an alkoxy group (OR) bonded to the anomeric carbon.
For example, treatment of α- or β-D-glucose with methanol and HCl forms two anomeric glycosides where the OR group is now pointing up and down:
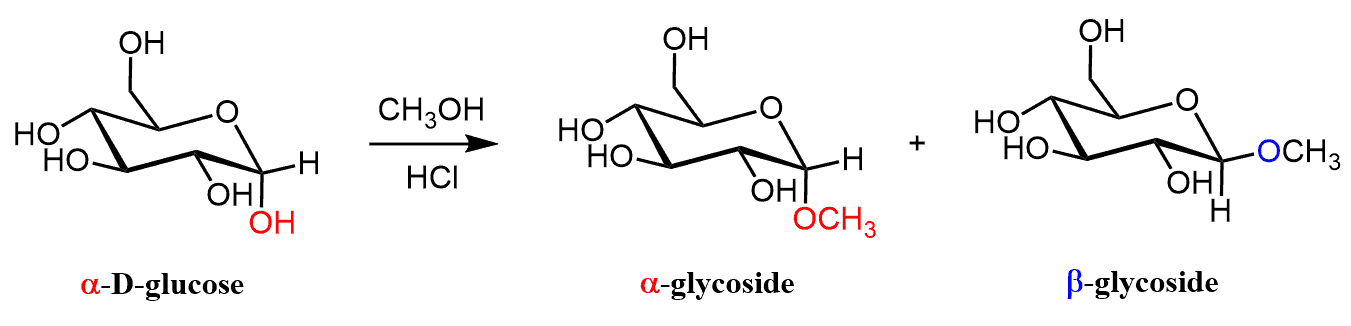
Let’s now understand why starting, for example with α -D-glucose, two diastereomers are formed rather and retaining or changing the configuration of the anomeric carbon. In the first step, the anomeric hydroxyl group is protonated and expelled by the lone pairs of the neighboring oxygen in the ring.
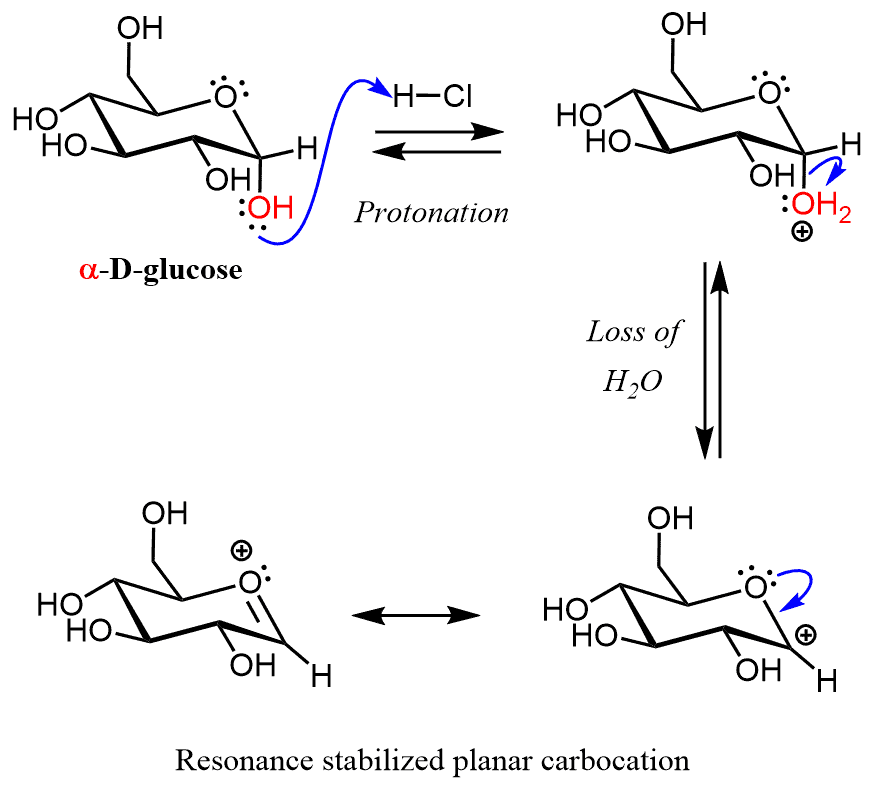
The resulting carbocation is resonance stabilized and this explains why out of all the OH groups of the sugar, only the one on the anomeric carbon reacts. Notice that in both resonance structures the there is a planar carbocation and this geometry allows for a nucleophilic attack by MeOH from both faces and therefore, two isomers are formed:
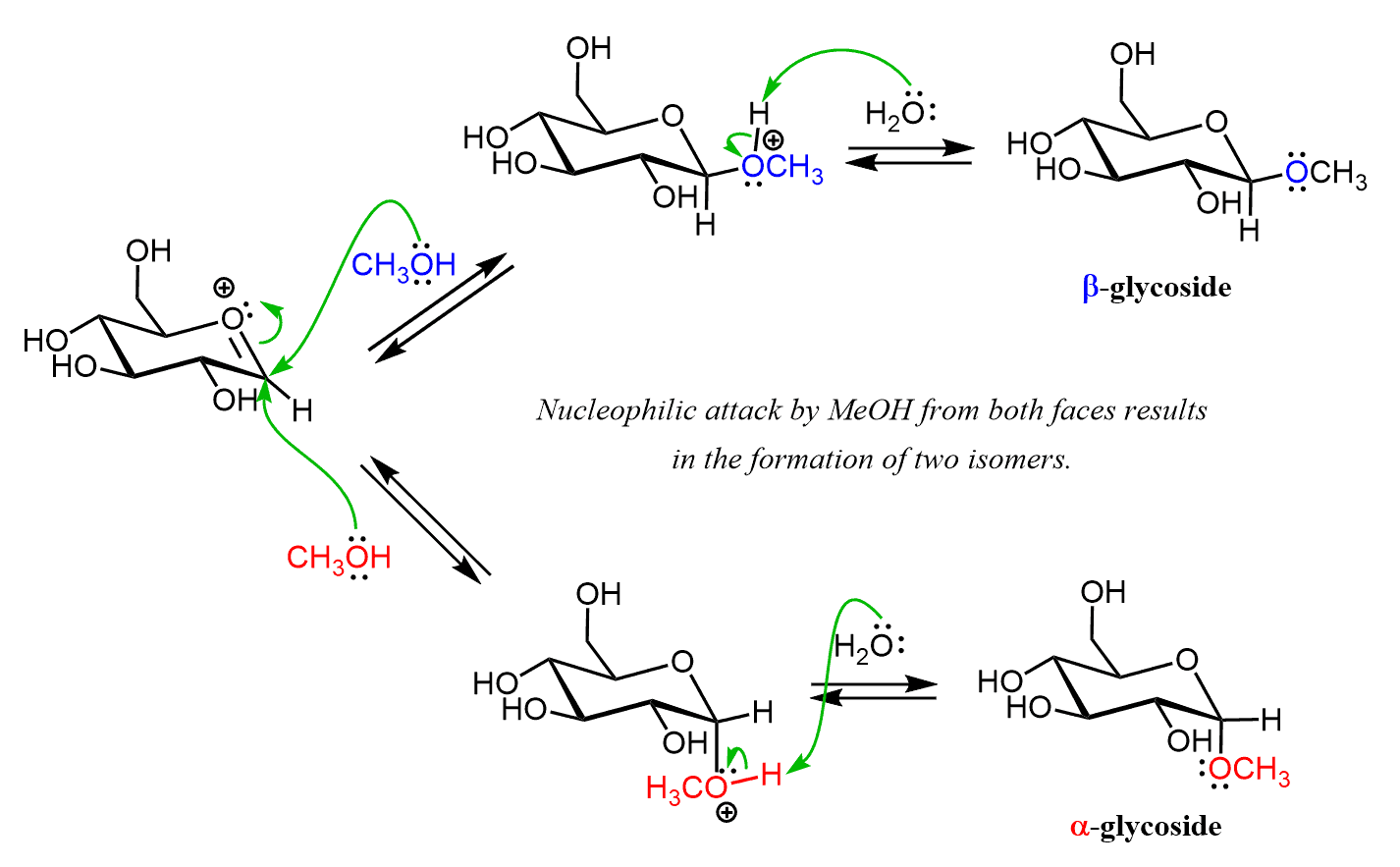
The glycosides are stable under neutral or basic conditions and unlike cyclic hemiacetals, they do not undergo mutarotation.
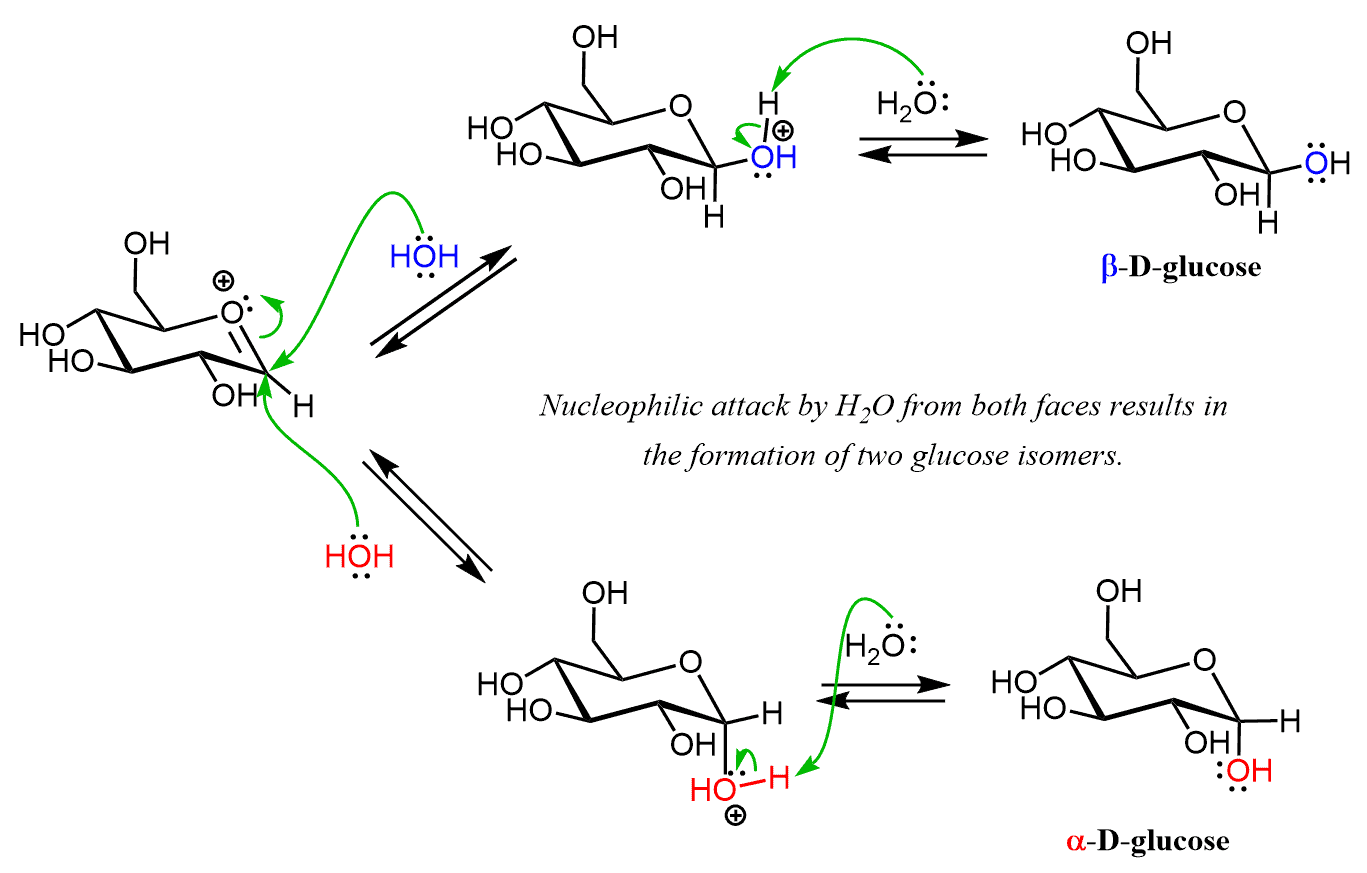
Glycoside Hydrolysis
Glycosides are stable in neutral/basic conditions and can be isolated and crystallized. The stability of acetals allows for their use as protecting groups for aldehydes and ketones in reactions involving strong bases and nucleophiles. In the contrary, the acetal protecting group is removed by an acidic treatment, so they can be hydrolyzed with acid and water back to cyclic hemiacetals. Just like in the formation of glycosides, a mixture of two anomers is formed from either glycoside:

The hydrolysis starts with the protonation of the alkoxy group on the anomeric carbon followed by the formation of a planar carbocation.
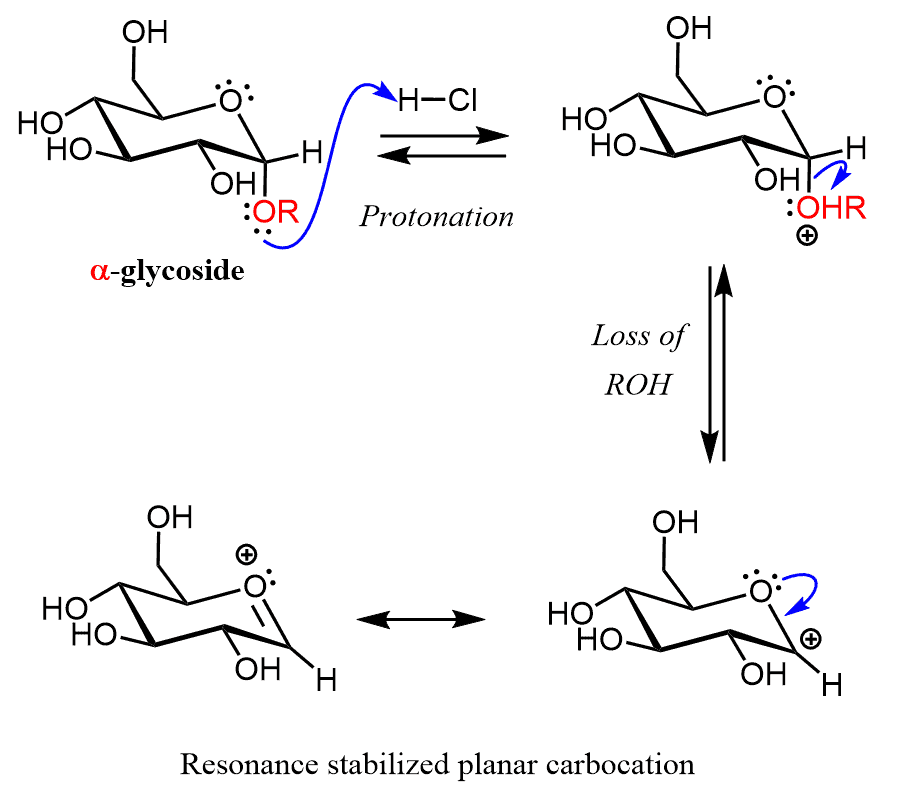
After the nucleophilic attack by water and a deprotonation step, the two isomers of glucose are formed. So, this mechanism is the reverse of glycoside formation, and the equilibrium in both cases is controlled by the large of the excess of the reactant.
Need some practice on carbohydrates?
Check this Multiple-Choice, summary quiz on the structure and reactions of carbohydrates with a 40-min video solution!
Check also in Carbohydrates
- Carbohydrates – Structure and Classification
- Erythro and Threo
- D and L Sugars
- Aldoses and Ketoses: Classification and Stereochemistry
- Epimers and Anomers
- Converting Fischer, Haworth, and Chair forms of Carbohydrates
- Mutarotation
- Glycosides
- Isomerization of Carbohydrates
- Ether and Ester Derivatives of Carbohydrates
- Oxidation of Monosaccharides
- Reduction of Monosaccharides
- Kiliani–Fischer Synthesis
- Wohl Degradation


