- 2. POLYAMIDE
Presented By:
Dilshad Bajwa
Ayesha Hameed
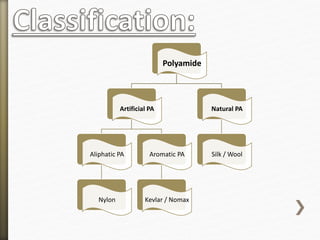
- 6. Polyamide
Artificial PA
Aliphatic PA
Nylon
Aromatic PA
Kevlar / Nomax
Natural PA
Silk / Wool
- 8. Nylon => Developed in the 1930’s at
DuPont by Wallace Carothers and
his team of researchers,
Kevlar => In the early 1960s, DuPont
was interested in developing
“super fibers” due to the prior valuable
invention of nylon,
In 1965, research scientist
Stephanie Kwolek from DuPont
discovered “kevlar”
Nomex=> material developed in the
early 1960s by DuPont and first marketed
in 1967,
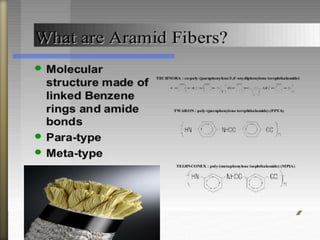
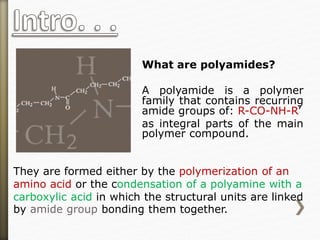
- 9. What are polyamides?
A polyamide is a polymer
family that contains recurring
amide groups of: R-CO-NH-R’
as integral parts of the main
polymer compound.
They are formed either by the polymerization of an
amino acid or the condensation of a polyamine with a
carboxylic acid in which the structural units are linked
by amide group bonding them together.
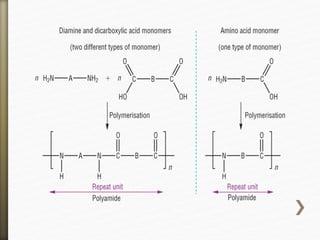
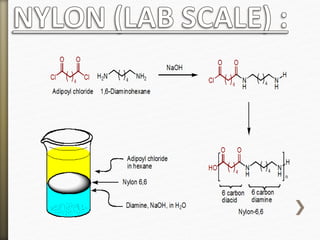
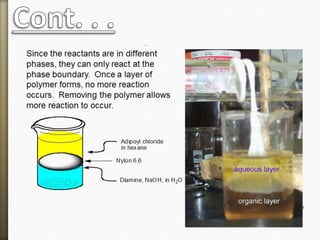
- 11. Nylon is formed by the condensation reaction of
two components:
Diamine (a compound containing two amino
[NH2] groups—e.g., hexamethylenediamine)
Dicarboxylic acid (containing two carboxyl
[CO−OH] groups—e.g., adipic acid),
Or may be formed by the self-condensation of an
amino acid or an amino-acid derivative.
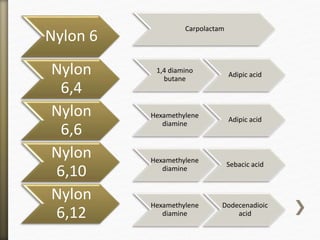
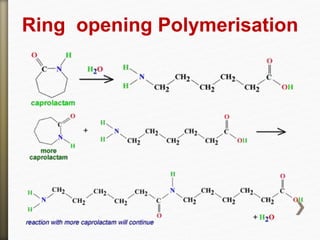
- 13. Nylon 6
Carpolactam
Nylon
6,4
1,4 diamino
butane
Adipic acid
Nylon
6,6
Hexamethylene
diamine
Adipic acid
Nylon
6,10
Hexamethylene
diamine
Sebacic acid
Nylon
6,12
Hexamethylene
diamine
Dodecenadioic
acid
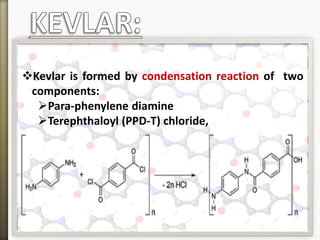
- 14. Kevlar is formed by condensation reaction of two
components:
Para-phenylene diamine
Terephthaloyl (PPD-T) chloride,
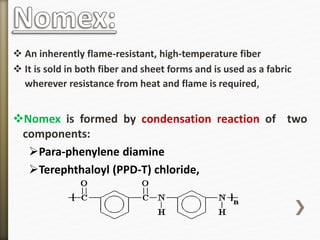
- 15. An inherently flame-resistant, high-temperature fiber
It is sold in both fiber and sheet forms and is used as a fabric
wherever resistance from heat and flame is required,
Nomex is formed by condensation reaction of two
components:
Para-phenylene diamine
Terephthaloyl (PPD-T) chloride,
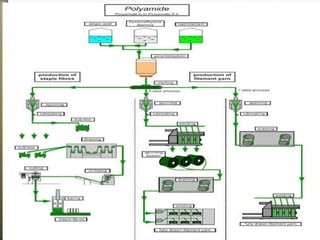
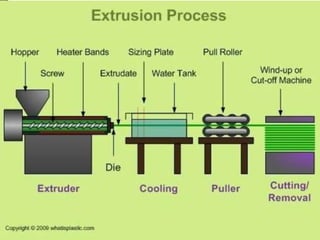
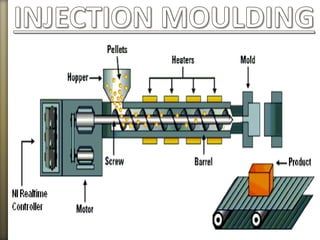
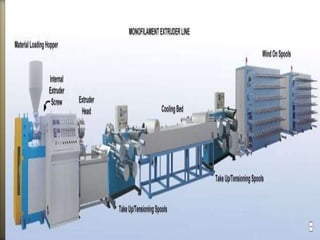
- 29. Solid nylon can be manufactured by:
Extrusion
Injection molding
Casting
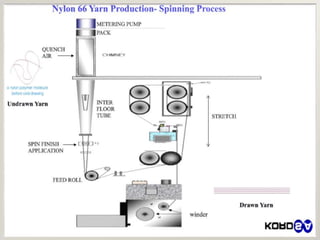
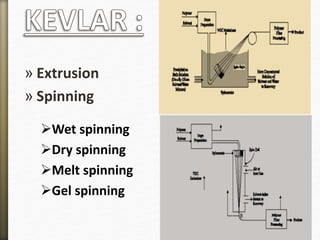
- 33. » Extrusion
» Spinning
Wet spinning
Dry spinning
Melt spinning
Gel spinning
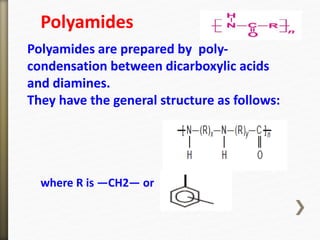
- 41. Polyamides are prepared by poly-
condensation between dicarboxylic acids
and diamines.
They have the general structure as follows:
where R is —CH2— or
Polyamides
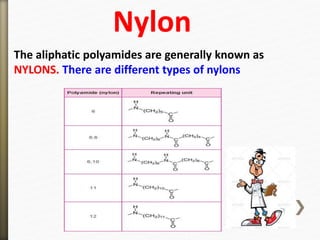
- 42. The aliphatic polyamides are generally known as
NYLONS. There are different types of nylons
Nylon
- 43. .
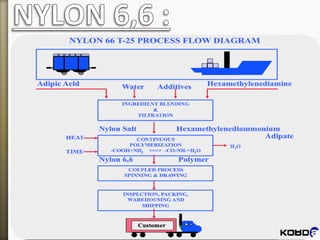
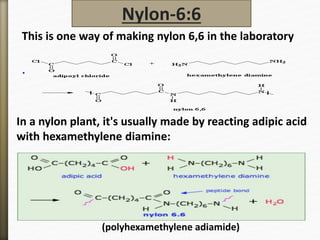
Nylon-6:6
This is one way of making nylon 6,6 in the laboratory
In a nylon plant, it's usually made by reacting adipic acid
with hexamethylene diamine:
(polyhexamethylene adiamide)
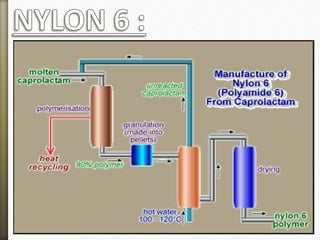
- 44. Nylon 6 is only made from one kind of monomer
called caprolactam. It's made by a ring opening
polymerization and self condensation
polymerization.
Slef Condensationion Polymerization:
Nylon 6
- 45. Ring opening Polymerisation
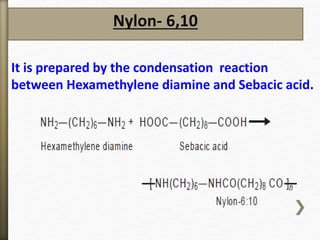
- 46. Nylon- 6,10
It is prepared by the condensation reaction
between Hexamethylene diamine and Sebacic acid.
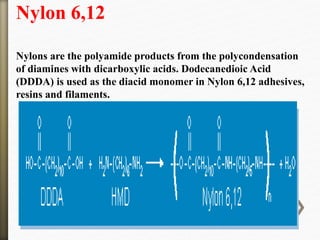
- 47. Nylon 6,12
Nylons are the polyamide products from the polycondensation
of diamines with dicarboxylic acids. Dodecanedioic Acid
(DDDA) is used as the diacid monomer in Nylon 6,12 adhesives,
resins and filaments.
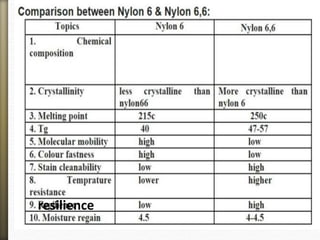
- 48. resilience
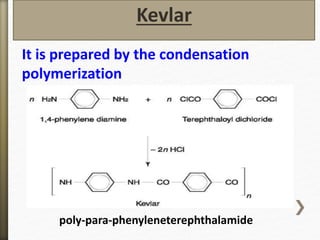
- 51. It is prepared by the condensation
polymerization
Kevlar
poly-para-phenyleneterephthalamide
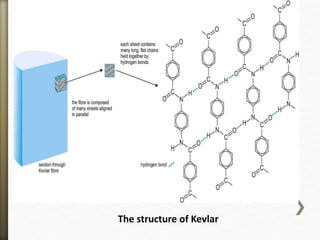
- 52. The structure of Kevlar
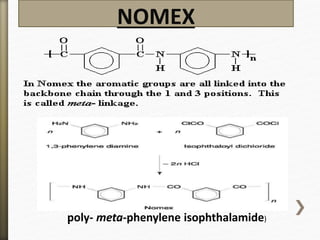
- 53. NOMEX
.poly- meta-phenylene isophthalamide)
- 56. Variation of luster: nylon has
the ability to be very lustrous,
semi lustrous or dull,
Durability and high elongation,
Excellent abrasion resistance,
High resistance to insects, fungi,
molds and many chemicals,
Melts instead of burning,
Good specific strength,
Absorbs more water,
- 57. » High strength to weight ratio.
» Low ductility.
» High modulus of rigidity (structural rigidity).
» Low electrical conductivity.
» High chemical resistance.
» Low Thermal Shrinkage
» High toughness (work-to-break).
» Excellent dimensional stability.
» Low machinability.
» Flame retardant, self-extinguishing
- 58. Heat and Flame Resistant
High Ultraviolet Resistance
High Chemical Resistance
Low Thermal Shrinkage
Formable for Molded Parts
Low Elongation to Break
- 60. TEXTILES
- 61. Nylon insulation on electric wires are used due to
its solvent ,wear and abrasion resistance.
- 62. Nylon Bike Made Using Satellite
Technology is as Strong as Steel!
- 65. Nylon Pipe Fitting Tube Nylon Pipes
Industrial Applications
- 68. safety airbags
Seat belts
- 69. HOUSEHOLD APPLICATIONS
- 70. Ropes made from polyamides are used by rock and ice climbers.
- 72. Kevlar
Kevlar is a very strong material - about five times
as strong as steel,
- 73. Kevlar used extensively in the manufacture of
panels and wings for fighter jets..
- 74. •Kevlar® 49 and
–High-modulus type used primarily in fiber optic
cable, ropes, cables, and composites for marine
applications.
Kevlar® 100
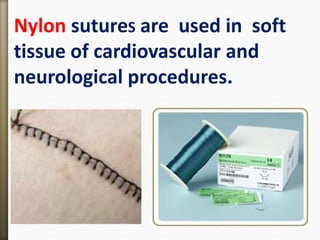
- 78. Nylon sutureS are used in soft
tissue of cardiovascular and
neurological procedures.







![Nylon is formed by the condensation reaction of
two components:
Diamine (a compound containing two amino
[NH2] groups—e.g., hexamethylenediamine)
Dicarboxylic acid (containing two carboxyl
[CO−OH] groups—e.g., adipic acid),
Or may be formed by the self-condensation of an
amino acid or an amino-acid derivative.](https://image.slidesharecdn.com/20-11-141218115404-conversion-gate02/85/polyamides-11-320.jpg)